 Choose language
Choose language
< Return to main menu
 Choose language
Choose language


In chapters 10 and 35, we discussed the use of QM analyses to rationalize regioselectivity observed in nucleophilic aromatic substitution (SnAr) of polyhalogenated substrates[1]. This type of reaction is usually mechanistically simple, operationally straightforward, and quite reproducible. Yet occasionally we do run into cases that are very sensitive to subtle variations, as exemplified by the reaction between 4-nitrobenzonitrile and sodium methoxide described by Ma in ChemRxiv[2] (Figure 1). He found that
1.Addition of 1 eq of MeONa gave no conversion of 1 to 2;
2.Conversion improved with excess of MeONa; with 10 eq of MeONa, reaction did not go to completion, with~30% of 1 remained;
3.Addition of a small amount of methanol drastically improved conversion;
4.But conversion dropped sharply with more than 40 eq of methanol;
5.All reactions gave a clean mixture of 1 and 2, with no other detectable byproducts.
In this chapter, we will use QM analyses to understand this interesting reaction, to account for the observations reported.
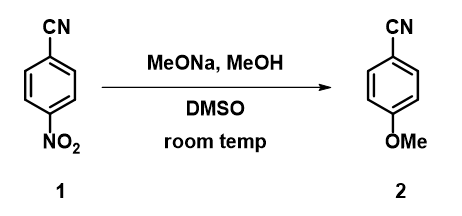
Figure 1. SnAr reaction between 4-nitrobenzonitrile and sodium methoxide
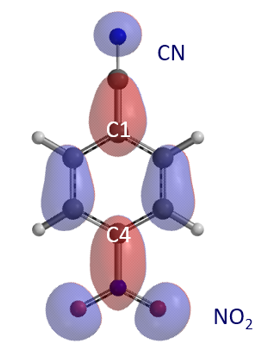
Figure 2. LUMO of 4-nitrobenzonitrile
Shown in Figure 2 is LUMO of 1 with significant lobes on C4 and C1, reaction centers for nucleophilic substitution. Reaction energy profile calculated for nucleophilic attack at C4 shows i) As methoxide approaches C4 carbon from 3.4 Å to 2.5 Å, energy of the reacting system drops by 6.06 kcal/mol; ii) The nucleophilic addition step has a relatively low energy barrier of 2.59 kcal/mol, suggesting that the reaction will proceed smoothly and thoroughly with one equivalent of sodium methoxide, which is inconsistent with observation 1.
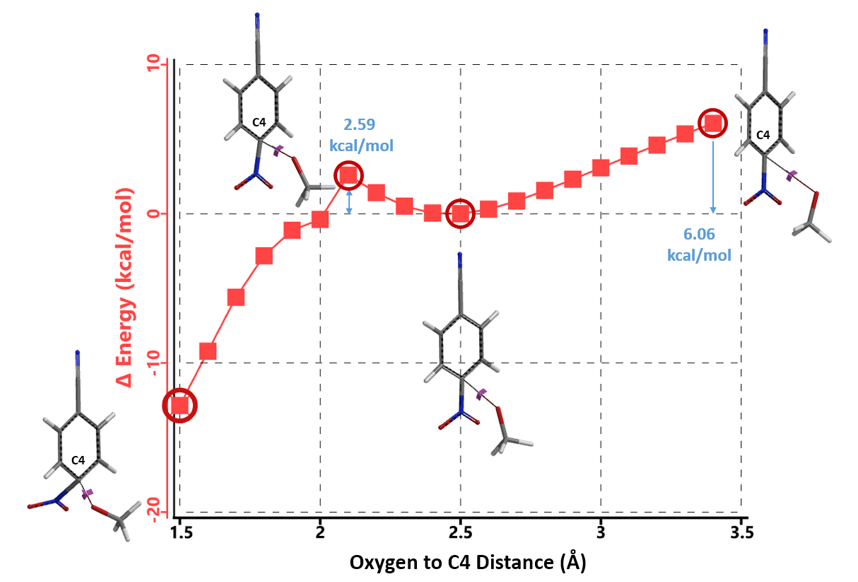
Figure 3. Reaction energy profile calculated for nucleophilic attack at C4
We then calculated for reaction energy profile for nucleophilic attack at C1 (Figure 4). As methoxide approaches C1 (3.4 Å to 2.4 Å), it gets closer to the nitrile carbon (3.25 Å to 1.48 Å), suggestive of formation of a covalent bond. Furthermore, the bond angle between cyanide group and C1 carbon also changes from 176.5º to 127.2º, indicating that the carbon-nitrogen triple bond of cyanide group is converting to the carbon-nitrogen double bond of an imidate. These changes are associated with an energy drop of 18.87 kcal/mol. Subsequent conversion from the imidate to 4-methoxybenzonitrile 2 (2.4 Å to 1.5 Å) will face a very high energy barrier of 35.93 kcal/mol, not consistent with a room temperature reaction.
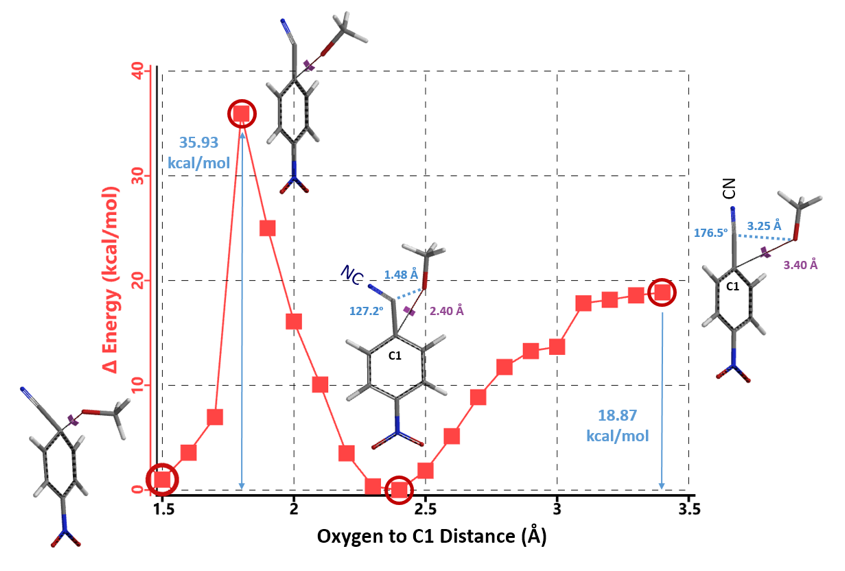
Figure 4. Reaction energy profile calculated for nucleophilic attack at C1
Based on the above calculation results, we reasoned that the imidate is likely to be an intermediate in this reaction. Direct reaction energy profile calculation for its formation suggests an even lower energy barrier of 0.78 kcal/mol (Figure 5) versus 2.59 kcal/mol for reaction at C4.
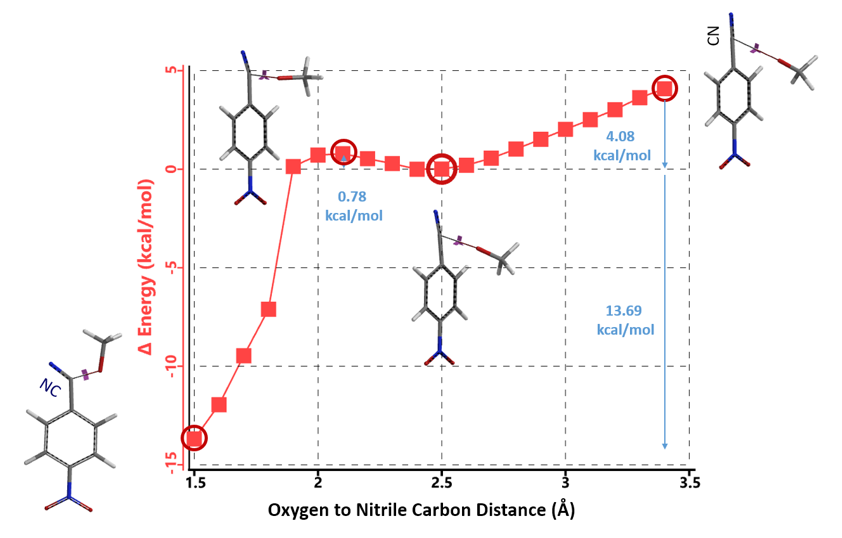
Figure 5. Reaction energy profile calculated for nucleophilic attack at nitrile group
This initial set of calculations enables us to conclude that the first equivalent of sodium methoxide will add on to the nitrile group of 1 to form intermediate 3, instead of displacing the nitro group to provide 2. This is consistent with observation 1, that is, when only one equivalent of sodium methoxide is added, no conversion of 1 to 2 could be detected.
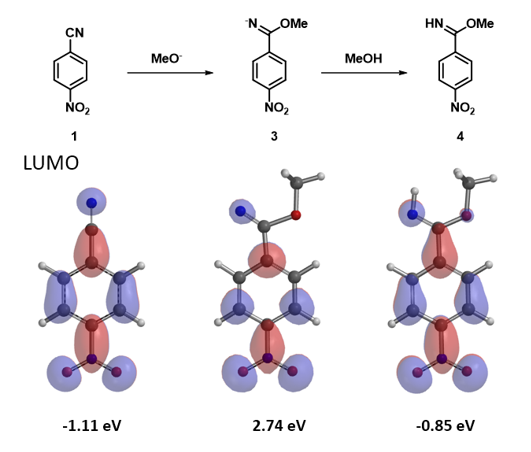
Figure 6. LUMO of 4-nitrobenzonitrile and corresponding imidates
LUMO energy calculated for the resultant imidate anion 3 is 2.74 eV, indicating that the electrophilicity of this intermediate is low (Figure 6). We reasoned that when a small amount of methanol is added, proton exchange will occur to provide the neutral imidate 4, with LUMO energy of -0.85 eV, electrophilic enough for further nucleophilic reaction. This could account for observation 3, that is, addition of a small amount of methanol drastically improved conversion.
Next we compared reaction energy profiles calculated for methoxide nucleophilic displacement of nitro group at C4 (Figure 7) versus addition to imidate group at C1 (Figure 8) with intermediate 4. Activation energies were found to be 4.54 kcal/mol versus 3.26 kcal/mol, respectively, with a difference of 1.28 kcal/mol, in favor of the addition to imidate group.
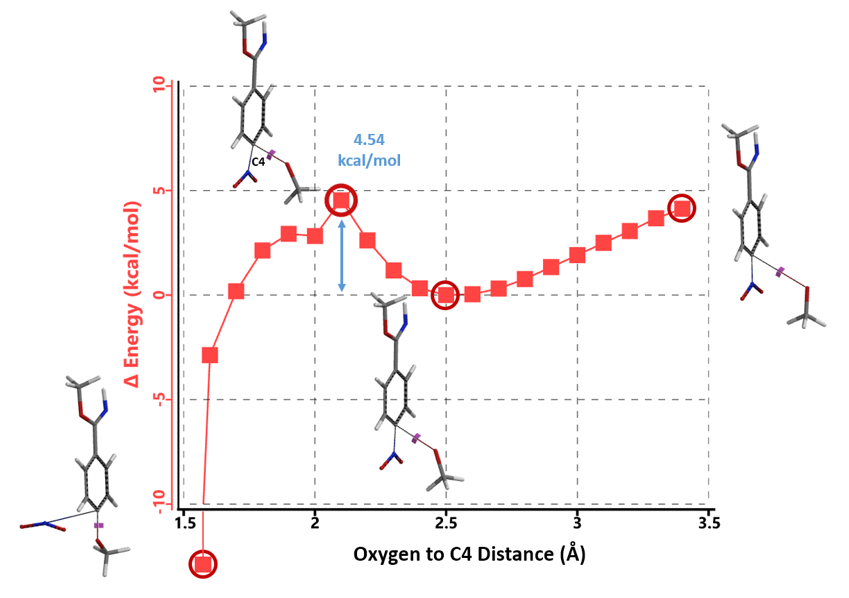
Figure 7. Reaction energy profile calculated for nucleophilic attack at C4
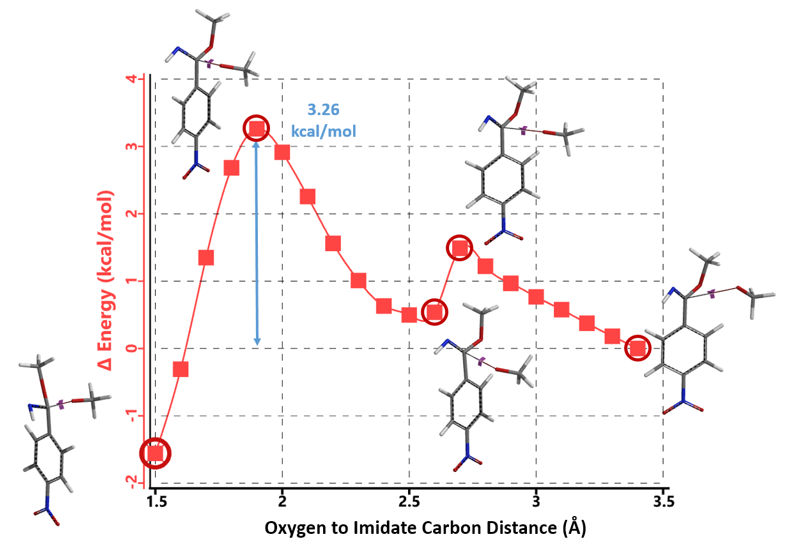
Figure 8. Reaction energy profile calculated for addition to imidate group
Similar to the conversion of intermediate 3 to 4, we reasoned that the resultant intermediate 5 will undergo proton exchange with methanol to form the neutral intermediate 6 (Figure 9), with a LUMO energy of -0.67 eV, electrophilic enough for reaction with the third equivalent of methoxide, for displacement of the nitro group.
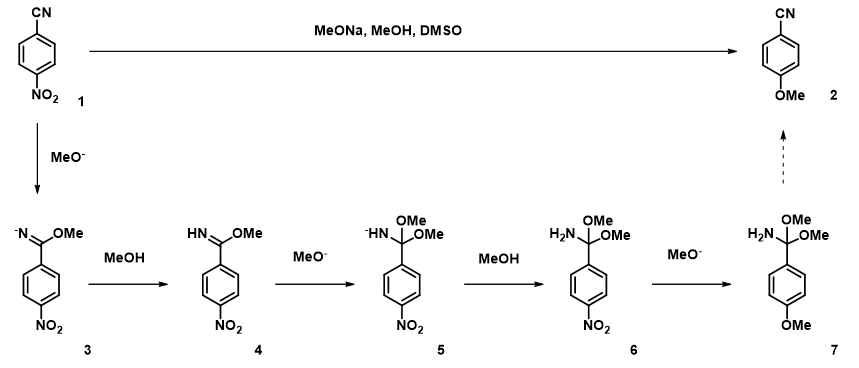
Figure 9. Proposed reaction mechanism with multiple nucleophilic reactions
With intermediate 6, we have only one electrophilic center to consider. Remarkably, the reaction energy profile calculated for methoxide displacement of the C4 nitro group exhibits two energy peaks (Figure 10), with oxygen to C4 distance of 2.1 Å and 1.8 Å, respectively. Follow-up calculation for the 2.4 Å to 1.6 Å portion of the energy profile at higher resolution, with a step size of 0.05 Å (Figure 11), shows clearly the presence of two energy peaks, one at 2.15 Å and one at 1.85 Å.
Transition state calculation of both structures revealed that only the structure at 1.85 Å has an imaginary frequency at i331 cm-1 which corresponds to the bonds being made and broken, satisfying key computational chemistry criteria as transition state (Figure 12). And the result provides an estimated activation energy of 5.59 kcal/mol for this rate limiting step, consistent with a room temperature reaction[3].
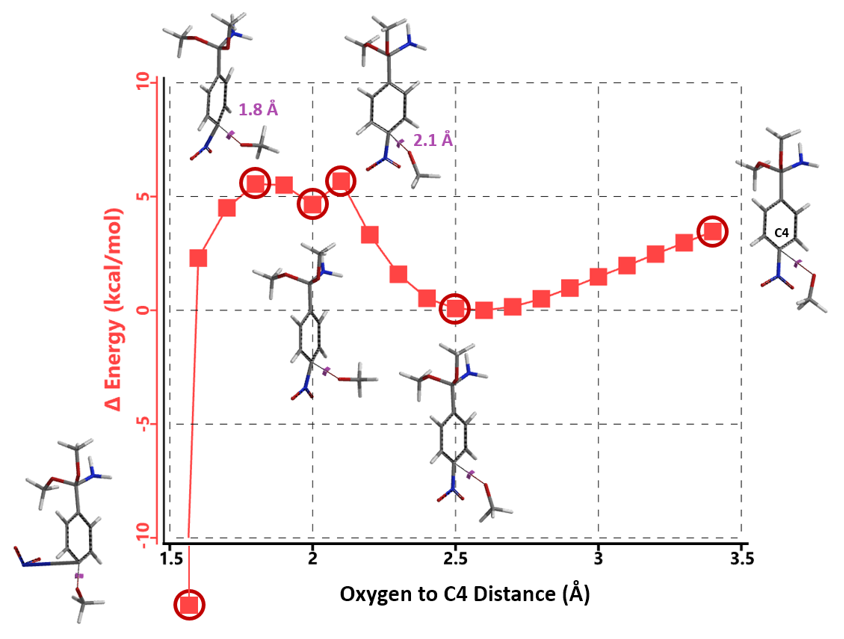
Figure 10. Reaction energy profile calculated for nucleophilic attack of intermediate 6 at C4
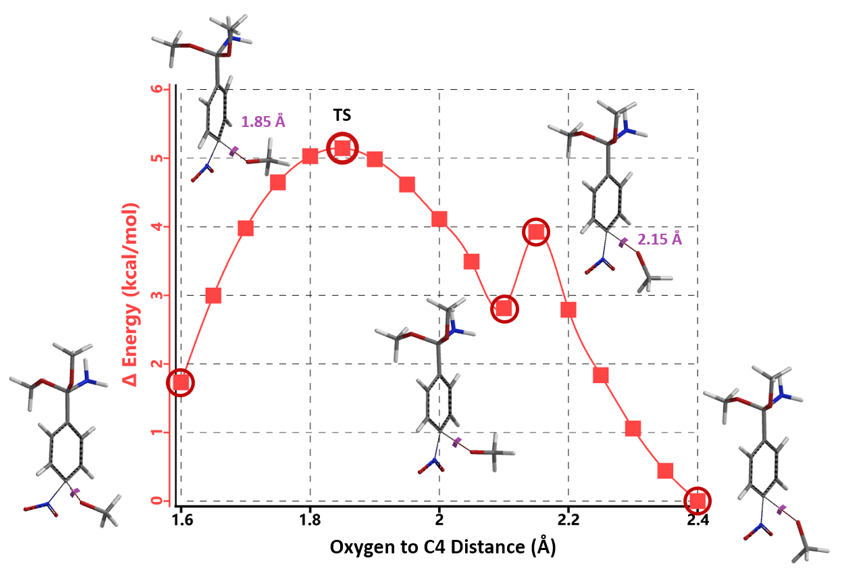
Figure 11. Reaction energy profile between 2.4 Å and 1.6 Å with a step size of 0.05 Å
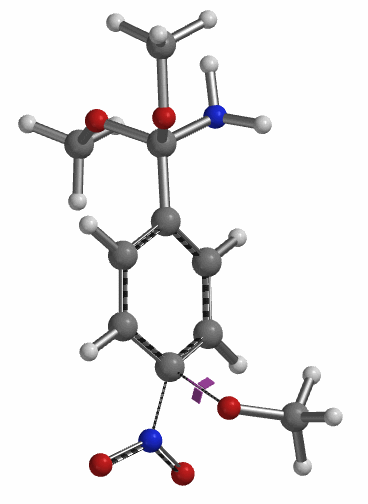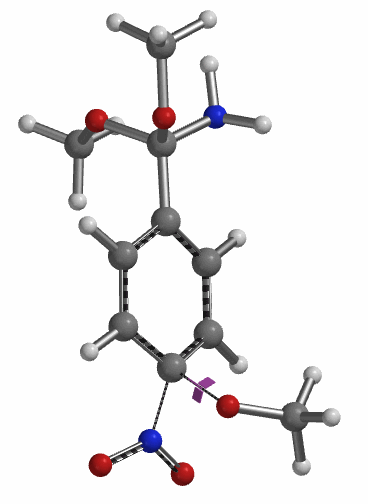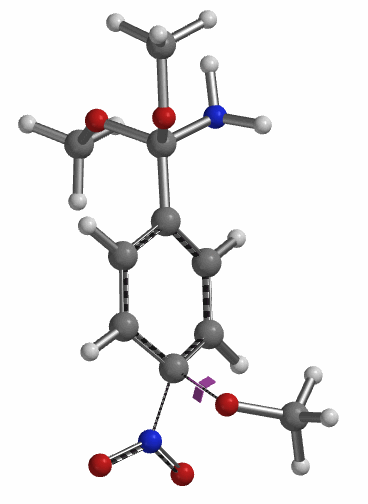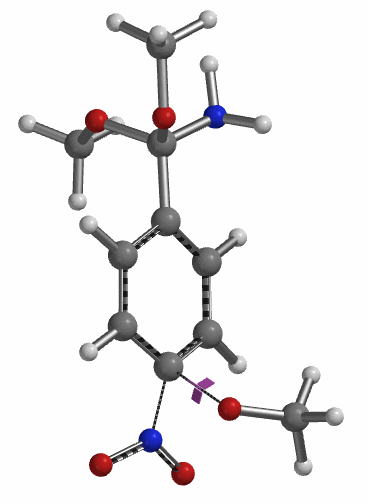
Figure 12. Transition State for third methoxide addition with an imaginary frequency of i331 cm-1
Our QM analyses are supportive of a reaction mechanism with three consecutive additions of methoxide, first to 4-nitrobenzonitrile 1, then to the resultant imidate intermediate 4, and finally to the intermediate 6, with twice proton exchanges with methanol in between to restore electrophilicity (Figure 13). The proposed mechanism could account for the intriguing observations reported. Since the difference in activation energy for formation of 5 versus 8 in the second methoxide addition step is relatively small ( ΔΔΕ of 1.28 kcal/mol), going through 8 could exist as a very minor pathway for the overall reaction.
Figure 13. Proposed reaction mechanism for SnAr reaction of 4-nitrobenzonitrile with methoxide
Nucleophilic aromatic substitution (SnAr) is usually mechanistically simple, operationally straightforward, and quite reproducible. Yet there are examples which are very sensitive to subtle variations, as exemplified by the above reaction between 4-nitrobenzonitrile with methoxide. Detailed and accurate records are crucial for understanding and optimizing these reactions. Precise execution of experimental conditions as recorded is very important when repeating such reactions!
For transformation of intermediate 7 to 4-methoxybenzonitrile (2), what are likely mechanisms? This happens during reaction with the excess sodium methoxide, or during aqueous work-up[4]?
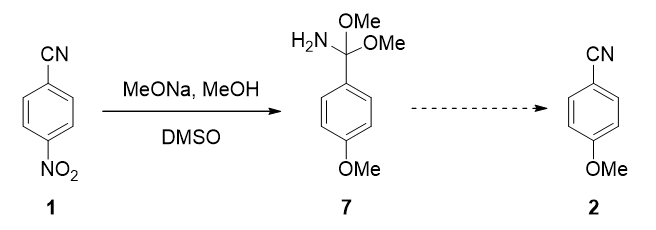
Figure 14. The last step in the reaction mechanism for conversion of 1 to 2
This article is written and edited by Yu Wang, Yongsheng Chen, John S. Wai
References:
[1] (a) QM chapter 10: SNAr reaction of polyhalogenated heterocycles.
(b) QM chapter 35: Highly regioselective SnAr of a polyhalogenated benzaldehyde
[2] Ma Y. Solvent Molecules Play a Role in an SNAr Reaction. ChemRxiv. Cambridge: Cambridge Open Engage; 2019; 10.26434/chemrxiv. 7782797. V1
[3] Spartan'20 Tutorial and User's Guide (2020). Irvine, CA, USA: Wavefunction, Inc. pp. 158, 442, 459 & 536 and Chapters 22 and 24 of the QM Organic Chemistry Class on the calculation of transition states and imaginary frequencies.
[4] Additional information to consider: 1) work-up: the reaction mixture was quenched with aqueous NaHCO3 and extracted with dichloromethane; 2) benzimidate reacts with water to form benzamide; 3) clean conversion of 4-nitrobenzonitrile to 4-methoxybenzonitrile, with no detectable byproducts.