Choose language
Choose language
< Return to main menu
 Choose language
Choose language

Services & Solutions

Services & Solutions
News & Events
Career
Platform
Small Molecule CRDMO

Small Molecule CRDMO
Oligonucleotide and Peptide CRDMO
C"R"DMO

C"R"DMO
CR"DM"O
Quantum Mechanics

Carbohydrate Chemistry
Nucleoside/tide
Macrocyclic Chemistry
Boron Chemistry
Steroids Chemistry
Nanomedicine
Computer-aided Drug Design
Library
Compound Management
Reaction Condition Screening
Fluorescent Bio-probe and Labeling
Quantum Mechanics
Late Stage Functionalization
Catalysis Screening
Chemical Synthesis Service
Discovery Process Chemistry
Bio-transformation
Impurity Proofing
Isotopic Labeling Chemistry
Crystallization Platform
Analysis & Purification
Fluorine Chemistry
Targeted Protein Degrader
Flow Chemistry Service
Electrochemistry
Photoredox Chemistry

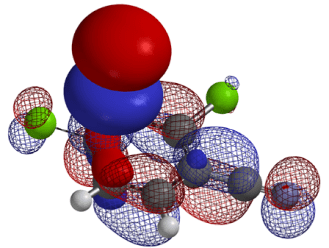
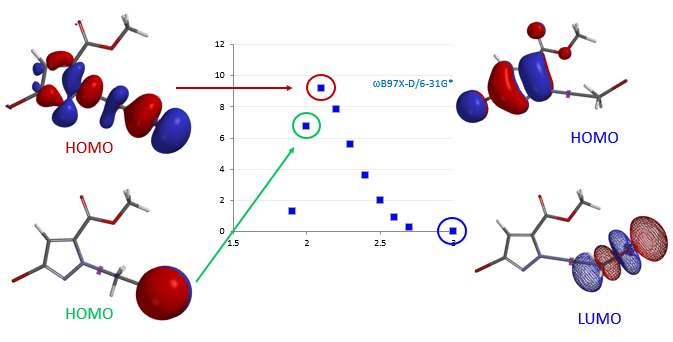
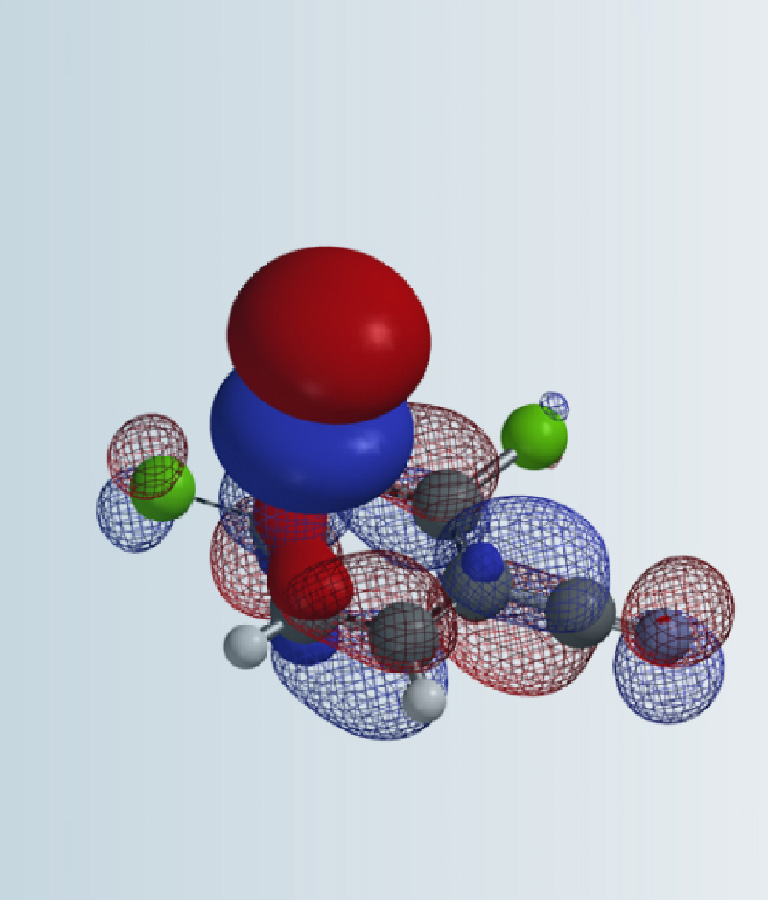
Synthesis of novel molecules continues to be a serious rate limiting step in chemistry discovery. Shortening the synthetic cycle time means investing only in reactions that are more likely to work throughout the synthetic sequence and have the least number of steps. Structural diversification, changes in substitution patterns, unique heterocyclic systems can often adversely impact the crucial step(s) of established synthetic sequences or development of feasible routes. Unique arrays of functionalities in novel heterocyclic systems can lead to unexpected reactivity. Retrospective Quantum Mechanics (QM) analysis of interesting and unanticipated observations showed that we could avoid most of these dead ends and provided insight on how to successfully overcome these synthetic challenges. Real-time incorporation of QM for prospective evaluations1 has greatly enhanced our success rates, reduced cycle times, and improved overall yield of our synthetic sequences.2
1 Glorius, F. et al, Chem. Soc. Rev., 2020, 49,6154. Nesse, F. et al. J. Am. Chem. Soc, 2019, 141, 2814.
2 Please visit “Magical Power of Quantum Mechanics” for further details.