 Choose language
Choose language
< Return to main menu
 Choose language
Choose language


In the previous QM chapters, we learned the concepts of LUMO and LUMO Map, and their application in analyses of organic chemistry reactions. In this chapter, we will apply these concepts on prospective analyses of a sequence of Cross Coupling Reactions, starting with a polyhalogenated pyrimidine.
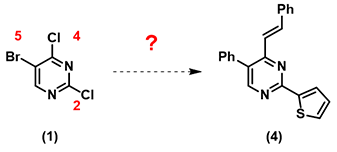
For a polyhalogenated benzene ring, reactivity towards oxidative addition usually follows the order I > Br > Cl. With polyhalogenated heterocyclic aromatic systems, experience taught us that it may not be that straightforward.
For 5-bromo-2,4-dichloropyrimidine (1), halogenated at position 2-, 4-, and 5-, which position is most labile towards oxidative addition? In palladium catalyzed cross-coupling reactions, we could consider Pd [0] as a nucleophile, as such, we first calculate for LUMO of (1) for prospective analysis.
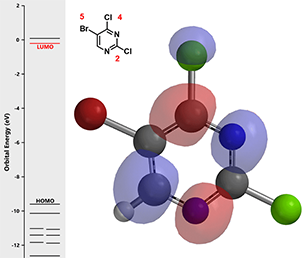
Figure 1. LUMO and associated energy of 5-bromo-2,4-dichloropyrimidine (1)
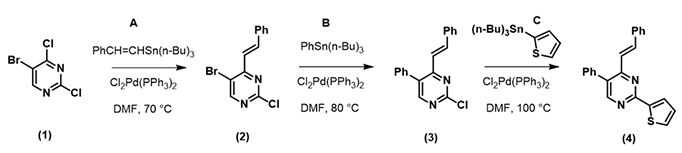
Calculated LUMO of starting material (1) reveals only the carbon at position 4, connecting to a chloro group, has a significant LUMO lobe associated with it (Figure 1). As such, under well controlled conditions, with reaction temperature maintained at 70 °C, pyrimidine (1) underwent selective cross coupling reaction with tin reagent A to provide C-4 vinyl intermediate (2) selectively.[2]
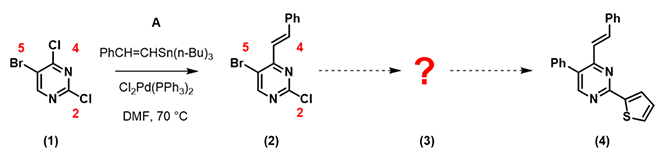
For the next step, again, we calculate the LUMO of intermediate (2) for analysis (Figure 2):
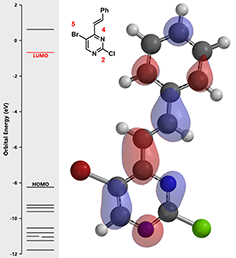
Figure 2. LUMO and associated energy of intermediate (2)
There are no obvious LUMO lobes on both carbon at C-2 and C-5 with a chloro and bromo substituents, respectively. In such situation, we learned to inspect the second lowest unoccupied molecular orbital, LUMO + 1 (Figure 3).
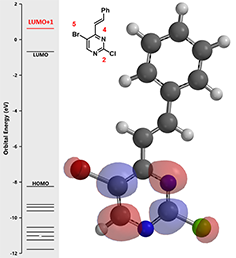
Figure 3. LUMO+1 and associated energy of intermediate (2)
LUMO+1 of pyrimidine intermediate 2 shows LUMO lobes at C-2 and C-5 positions, but no significant difference in their relative sizes. Does that mean there is no intrinsi c bias for the next oxidative addition? We know the answer is no. Then what will be the next trick up our sleeves?
Here we would like to introduce the use of QM calculated C-X bond infrared stretching vibrational frequencies to differentiate their relative reactivity toward oxidative addition. The weaker the C-X bond is, the lower the wave number it has[3], and the easier it is for the bond to undergo reaction.
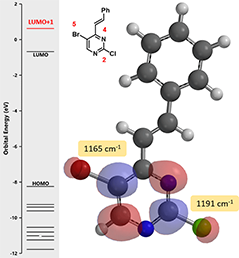
Figure 4. LUMO+1 and IR stretching frequencies of intermediate (2)
QM calculation indicated that the C-Br bond (1165 cm-1) is weaker than the C-Cl bond (1191 cm-1), and therefore the oxidative addition reaction is more likely to occur, consistent with experimental observation. It is worth noting that the lowest unoccupied orbital energy of starting material (1) is -0.21 eV, and the energy of LUMO + 1 of intermediate (2) is 0.62 eV. This suggests that we need to raise the reaction temperature for the second cross coupling reaction to proceed. Indeed, reaction of compound (2) with tin reagent B proceeded smoothly at 80 °C, with the same Cl2Pd(PPh3)2 catalyst as previous step, to provide the phenylated intermediate (3) as the single product.

In the last step, intermediate (3) does not have LUMO lobe on C-2, yet a very clear one with the higher energy LUMO +1.
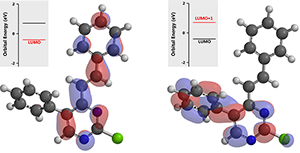
Figure 5.Schematic of LUMO (left) and LUMO + 1 (right) for compound (3)
With intermediate (3), since its LUMO + 1 orbital energy is higher than that of intermediate (2), the reaction temperature was raised further to 100 °C for the final cross coupling reaction to proceed.
With the above sequence of Cross Coupling reactions, using the same catalyst and solvent, one could envision running the whole sequence in one pot by adding corresponding tin reagents at different stages and raising the reaction temperature accordingly.
QM has proven to be a very powerful tool for organic chemists to analyze their synthetic schemes prospectively, and to evaluate objectively each of the reactions in the sequence.
In summary, for sequential Cross Coupling reactions of polyhalogenated heterocycles, we first look at relative sizes of LUMO or LUMO + 1 lobes on the carbons with halogen connecting to them. If the relative sizes of the lobes are similar, we compare the calculated C-X bond infrared stretching vibrational frequencies. These parameters enable us to sequence the order of reactions in a highly predictable manner.
Let’s apply what we learned above in 3-bromo-5,7-dichloropyrazolo[1,5-a]pyrimidine 5shown below.
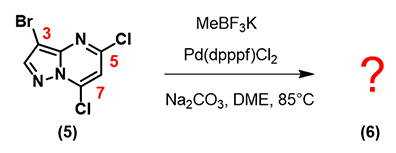
Based on the LUMO and calculated C-X bond stretching info, where will the first oxidative addition occur?
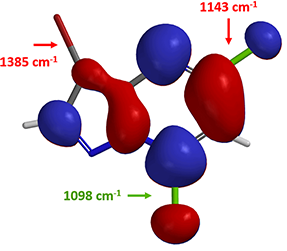
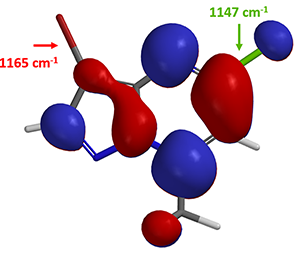
Yes, on the C-7 carbon – chloride bond. How about the next oxidative addition?
References:
[1] W. Hehre and S. Ohlinger, A Guide to Molecular Mechanics and Quantum Chemical Calculations. Irvine, CA, USA: Wavefunction, Inc., 2003.
[2] J.A. Joule & K. Mills, Heterocyclic Chemistry 4th Ed. Malden, MA, USA: Blackwell Publishing Ltd., 2000; pp 202-203.
[3] R.M. Badger, J. Chem. Phys. 1934, 2, 128.