 Choose language
Choose language
< Return to main menu
 Choose language
Choose language

In Chapter 5 “Sequential Cross Coupling Reactions of Polyhalogenated Heterocycles”, we discussed the use of QM calculated LUMO/LUMO map and IR carbon-halogen bond stretching wavenumbers to predict the order of haloselectivity for a sequence of palladium-catalyzed cross coupling reactions on monocyclic 2,4-dichloro-5-bromopyrimidine. We left a related question on predicting haloselectivity of palladium catalyzed cross coupling reaction for the bicyclic 3-bromo-5,7-dichloropyrazolopyrimidine (1). What will be the order of haloselectivity (Figure 1)?
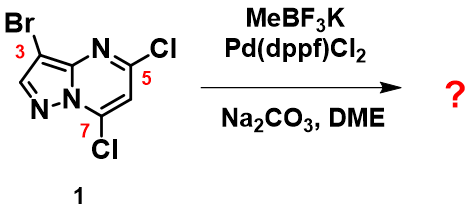
Figure 1. Suzuki Cross Coupling reaction of compound 1
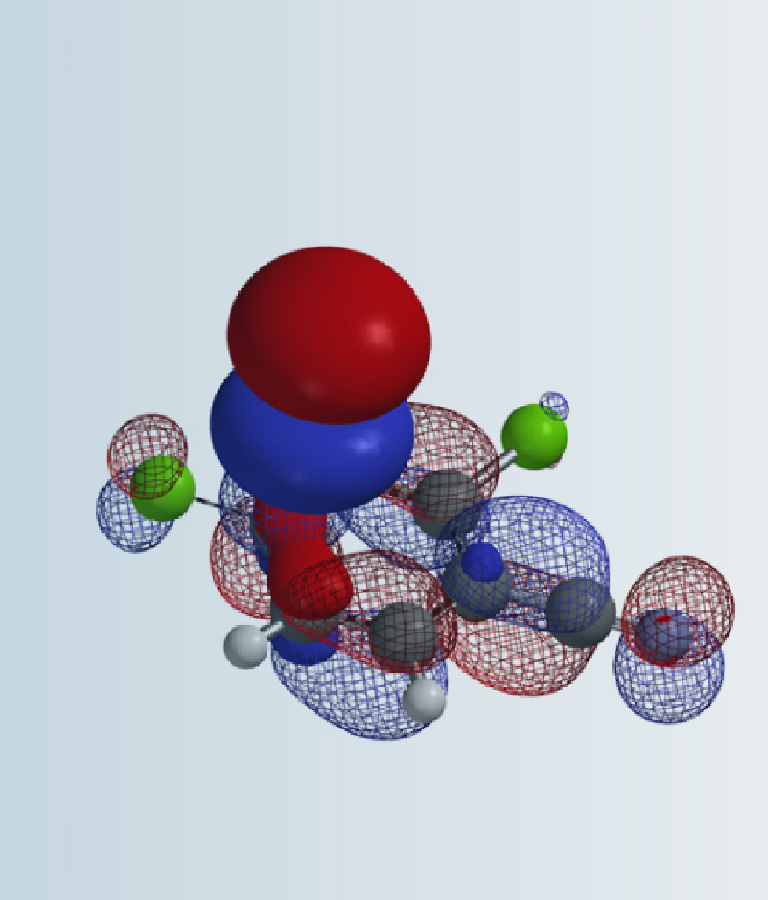
Shown in Figure 2 are the calculated LUMO and IR stretching vibration wavenumbers of the three carbon-halogen bonds of compound 1.
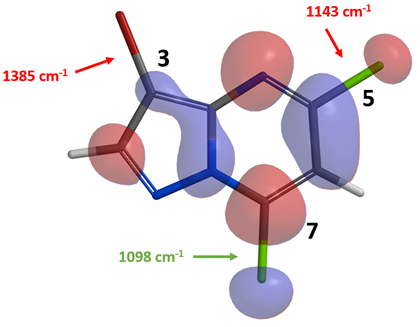
Figure 2. LUMO of compound 1 and IR stretching vibration wavenumbers of the C-X bonds
There is little LUMO lobe on the C3-Br carbon relative to the C5-Cl and C7-Cl carbons on compound 1. IR calculation shows that the stretching vibration wavenumbers of the C7-Cl and C5-Cl bonds are 1098 and 1143 cm-1, respectively, suggesting that the C7-Cl bond is weaker and will be more reactive toward Pd(0) oxidative addition. Our experimental result is consistent with the above prospective analysis, Suzuki cross coupling reaction of compound 1 with potassium methyl-trifluoroborate provides compound 2 selectively [1]. An obvious follow-up question is haloselectivity of intermediate 2.
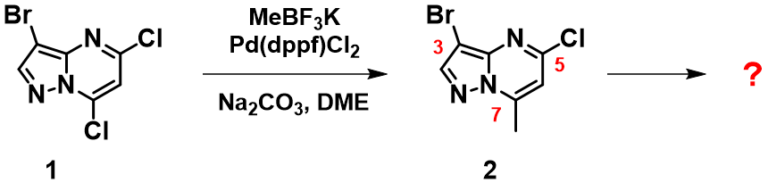
Figure 3. Suzuki Cross Coupling reaction of intermediate 1
With intermediate 2, there is little LUMO lobe on the C3-Br carbon relative to the C5-Cl one. IR calculation shows that the stretching vibration wavenumber of the C3-Br and C5-Cl bonds are 1168 and 1147 cm-1, respectively, suggesting that the C5-Cl bond is more reactive toward oxidative addition. Both LUMO and IR analyses are suggestive of selective reaction at C5-Cl, consistent with experimental results. Here we can see that the haloselectivity of metal-catalyzed coupling reactions of compounds 1 and 2 diverges from the general reactivity we learned from general organic chemistry for C-X bonds, i.e., X = I > Br ∼ OTf >> Cl >> F, yet fully accountable with relevant QM calculated parameters.
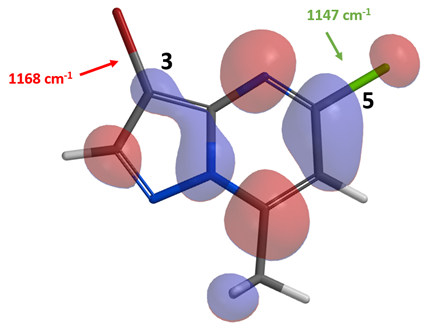
Figure 4. LUMO of intermediate 2 and IR stretching vibration wavenumbers of the C-X bonds
From the previous example, we learned that for certain bicyclic heterocyclic systems, C-Cl bonds could undergo oxidative addition with Pd(0) selectively over C-Br bonds. What have we learned about iodo containing polyhalogenated bicyclic systems? Below are two examples.
Figure 5. Buchwald reaction of aromatic heterocyclic compound 4
Shown in Figure 5 is 4-chloro-7-iodopyrrolotriazine 4, prepared by NIS iodination of commercially available chloropyrrolotriazine 3. Intuitively, we anticipated the C7-iodide bond to be more susceptible to oxidative addition to give selectively a C-7 amination product. However, only C-4 amination product was obtained under various Buchwald amination conditions. With compound 4, there is little LUMO lobe on the C7-I carbon relative to the C4-Cl one. IR calculation shows that the stretching vibration wavenumber of the C7-I and C4-Cl bonds are 873 and 823 cm-1, respectively, suggesting that the C4-Cl bond to be more reactive toward oxidative addition. Both LUMO and IR analyses are suggestive of selective reaction at C4-Cl, consistent with experimental observations. We learned to integrate these analyses to our retrosynthetic planning.
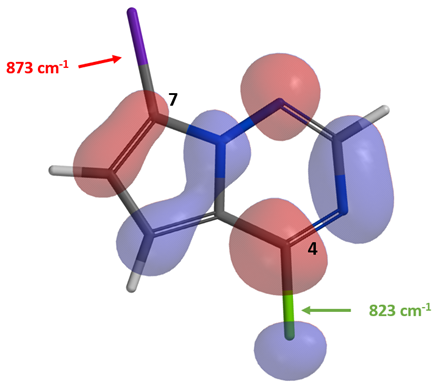
Figure 6. LUMO of compound 4 and IR stretching vibration wavenumbers of the C-X bonds
Second example comes from an OPRD paper published by Pfizer[2] (Figure 7). For nucleophilic aromatic substitution of 2,4-dichloro-5-iodopyrrolopyrimidine 5 with a secondary alcohol compound, we also calculated for its LUMO and C-X bond stretching wavenumbers. As shown in Figure 8, there are LUMO lobes on all three carbon-halogen bonds, yet with the lobe on C4-Cl carbon significantly larger than the ones on C5-I and C2-Cl carbons. IR calculation shows that the stretching vibration wavenumber of the C2-Cl, C4-Cl, and C5-I bonds are 981, 837, and 924 cm-1, respectively, suggesting that the C4-Cl bond is weakest and most reactive toward nucleophilic substitution, accounting for the high regioselectivity reported.
Figure 7. SnAr and Negishi reactions of polyhalogenated pyrrolopyrimidine 5
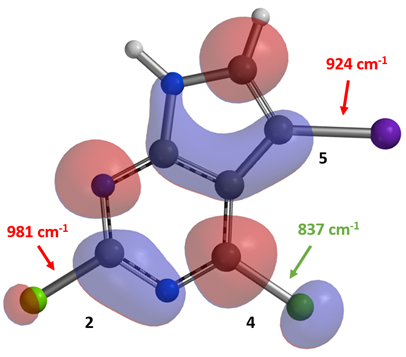
Figure 8. LUMO of compound 5 and IR stretching vibration wavenumbers of the C-X bonds
With polyhalogenated heterocycles, the C-X bonds’ relative reactivity towards metal-catalyzed coupling reactions does not necessarily follow the general reactivity order of X = I > Br ∼ OTf >> Cl >> F. In the case of bicyclic heteroaromatic systems, if the C-Cl bond is on the electron-deficient part of the system, while the C-I or C-Br bonds are on the electron-rich part, we shall calculate for LUMO and IR C-X bonds’ stretching vibration wavenumbers to sequence properly the order of reactions. These intrinsic changes in halo reactivity are predictable.
How about polyhalogenated tricyclic heteroaromatic substrates? Let’s analyze 5-bromo-9-chloropyridopyrropyrimidine (8). Based on the calculated LUMO and C-X bond stretching wavenumbers shown in Figure 9, will palladium-catalyzed reactions be selective on the C-Br or C-Cl bond? Which factor controls the selectivity observed for this substrate [3]?
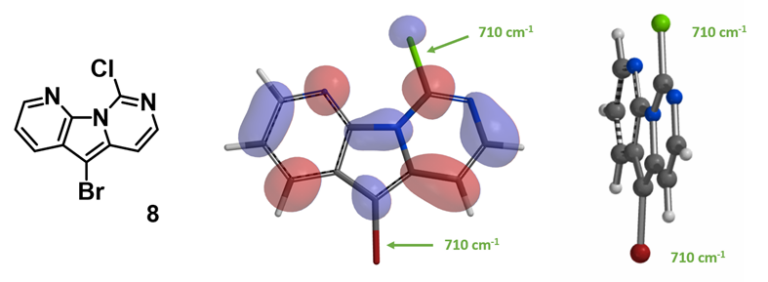
Figure 9. LUMO of compound 8 and IR stretching vibration wavenumbers of the C-X bonds
Zhong Zheng, Qiuyue Wang, Yongsheng Chen, John S. Wai
References:
[1] Z. Xu, Y. Lou, Fused heterocyclic compound, preparation method therefor, pharmaceutical composition, and uses therefore. US 20160244432 A1, August 25, 2016, page 28 on related Suzuki reaction with Compound 1.
[2] Y. Tao, N.F. Keene, K.E. Wiglesworth, B. Sitter, J.C. McWilliams, Org. Process Res. Dev. 2019, 23, 382.
[3] A. Baeza, J. Mendiola, C. Burgos, J. Alvarez-Builla, J. J. Vaquero, Eur. J. Org. Chem. 2010, 29, 5607.