 Choose language
Choose language
< Return to main menu
 Choose language
Choose language

This chapter covers four questions we had in an open-book QM examination in WuXi AppTec. For setting up QM calculations and interpreting results, participants have access to our QM e-book [1] and Spartan manual [2]. They were requested to refrain from doing literature search for answers during the 2-hour exam.
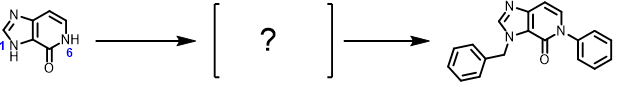
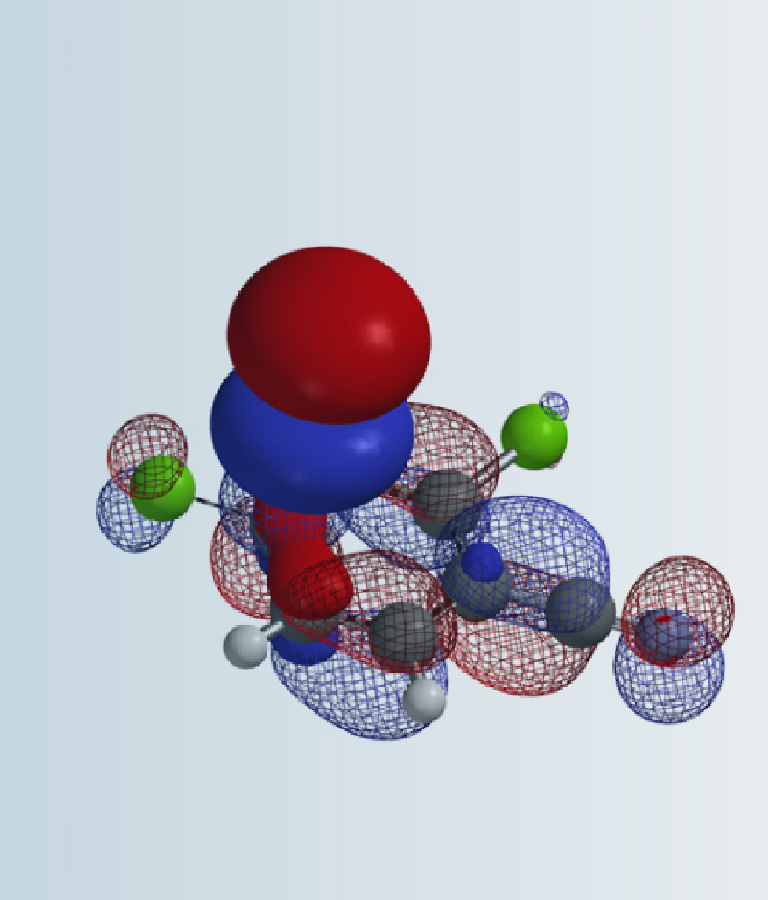
Which nitrogen shall be selectively functionalized first to attain the final compound? What will be the governing factor? What QM parameter to calculate for analysis? Please provide QM calculation results and interpretation.
Answer (Dong Pan)
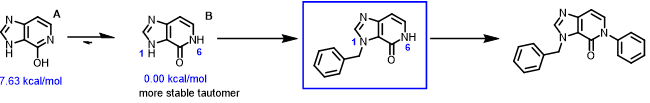
Figure 1. Relative energies of the two major tautomers of the substrate
The imidazopyridinone substrate could exist in several tautomeric forms, among which are tautomers A and B with relatively lower energy. The relative energy difference between the A enolic vs the B amide form is 7.63 vs 0.00 kcal/mol (Figure 1). As such, the substrate is 100% in the amide form and being used for the following analysis. The relative acidity of the two NH groups is the key factor in deciding for the proper order of reactions. This will require us to calculate for the electrostatic potential (ESP) map of structure B for comparison [4].
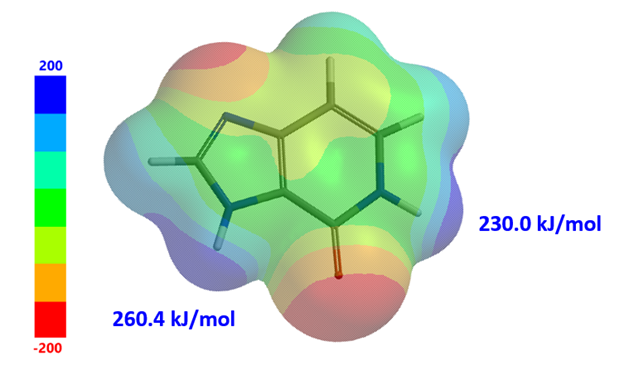
Figure 2. Electrostatic potential diagram of tautomer B
ESP map reveals N1-H and N6-H electric potential are 260.4 and 230.0 kJ/mol, respectively (Fig. 2). The larger the value, the stronger the acidity. With electrostatic potential of N1-H significantly higher than that of N6-H by 30 kJ/mol, we could selectively deprotonate N1-H for benzylation first [5].
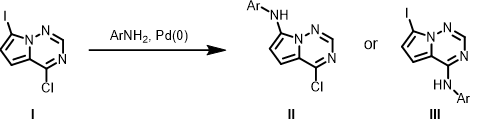
For the above Buchwald amination, will the reaction form a single product or mixture of products? If it is a single product, which one, II or III? What are the QM parameters to calculate for analysis? Please provide QM calculation results and interpretation.
Answer (Hailong Ren)
For the Buchwald amination of 4-chloro-7-iodopyrrolotriazine (I), we first examine LUMO of the substrate [6] (Figure 3). The LUMO lobe on C-Cl carbon is significantly larger than that on C-I carbon with the iodine carbon atom on the pyrrole, suggestive of selective oxidative addition (OA) and SnAr reaction at the C-Cl carbon [7]. LUMO+1 of I has a unique” string of pearls” lobes along the C-I bond, suggestive of selective metal halogen exchange reaction at the C-I carbon [8]. LUMO+1 does not have orbital lobe characteristic for OA and SnAr reactions, we do not need to consider it for Buchwald amination.
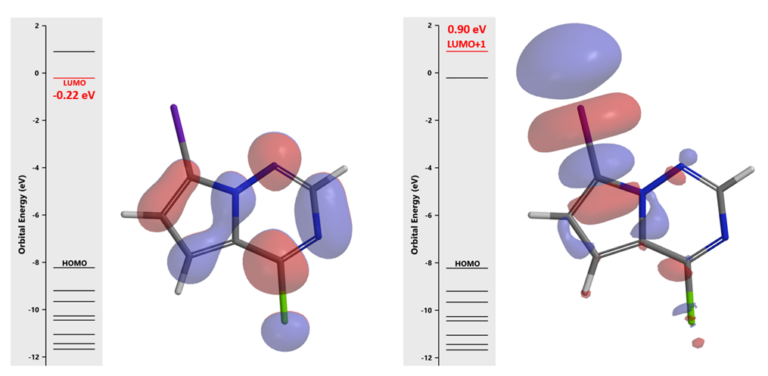
Figure 3. LUMO (left) and LUMO+1 (right) of pyrrolotriazine I
The prediction of C-Cl is more reactive than C-I towards OA is further supported with LUMO Map (Figure 4) and infrared (IR) stretching vibration frequencies of CX bonds. For substrate I, wavenumber calculated for C-Cl bond is 1191 cm-1 vs 1361 cm-1 for the C-I bond. The C-Cl bond is significantly weaker than the C-I bond. As such, results from LUMO and IR analyses are consistent, pointing towards a selective Buchwald amination at the C-Cl carbon.
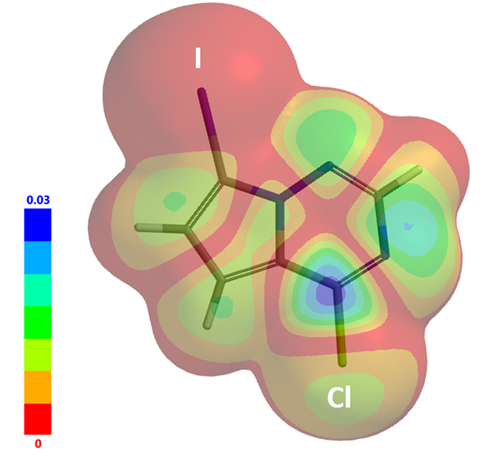
Figure 4. LUMO Map of substrate I
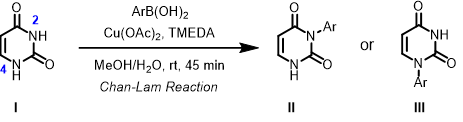
Will the above Chan-Lam reaction form a single product or mixture of products? If it is a single product, which one, II or III? What is the QM parameter to calculate for analysis? Please provide QM calculation results and interpretation.
Answer (Qiuyue Wang)
Quantum Mechanical Organic Chemistry chapter 34[10] showed us how to predict regio-selectivity for Chan-Lam reaction. We’ll find out whether N2-H or N4-H on uracil (I) is more acidic, and then examine HOMO of the deprotonated intermediate.
Electrostatic Potential Map of uracil (Figure 5, left) reveals that N4-H is more acidic than N2-H[4] (ESP at N4-H: 272.0 kJ/mol >> N2-H: 200.9 kJ/mol). N4-H will be selectively deprotonated under the basic conditions in Chan-Lam reaction. HOMO of the anionic intermediate (Figure 5, right) has HOMO lobe at N4, none at N2, enabling the prediction that Chan-Lam reaction of uracil (I) will occur preferentially at N-4 to provide a single product III.
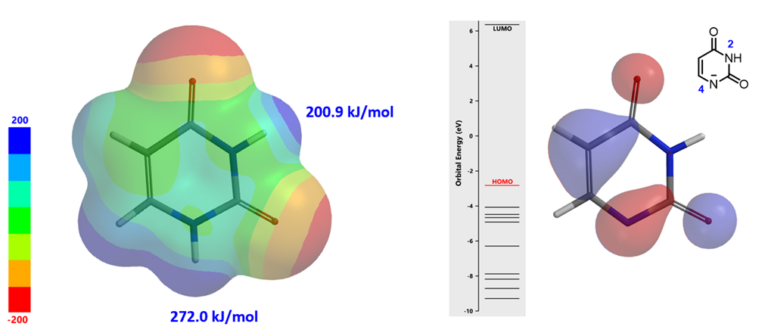
Figure 5. The ESP map of uracil (left) and HOMO of N4 deprotonated intermediate (right)
Will the above SNAr displacement reaction form a single product or mixture of products? If it is a single product, which one, II or III? What are the QM parameters to calculate for analysis? Please provide QM calculation results and interpretation.
Answer (Zhong Zheng)
For nucleophilic substitution reaction, we’ll look at unoccupied orbitals of pyridine I [6]. With LUMO, the lobe on C2 and C3 is mainly distributed between them, on the carbon-carbon bond (Figure 6, left). With LUMO+1, we have what we are looking for (Figure 6, right), lobes that are centered on the C-X carbons for further analysis.
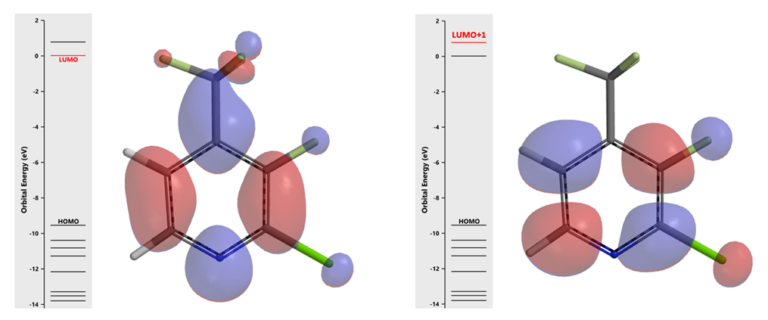
Figure 6. LUMO and LUMO+1 of compound I
LUMO+1 map (Figure 7, left) and LUMO+1/Electron density map (Figure 7, right) reveal that the LUMO lobe on C3-F carbon is more exposed and accessible than C2-Cl carbon for nucleophilic substitution reaction, suggestive of potential selective formation of product III.
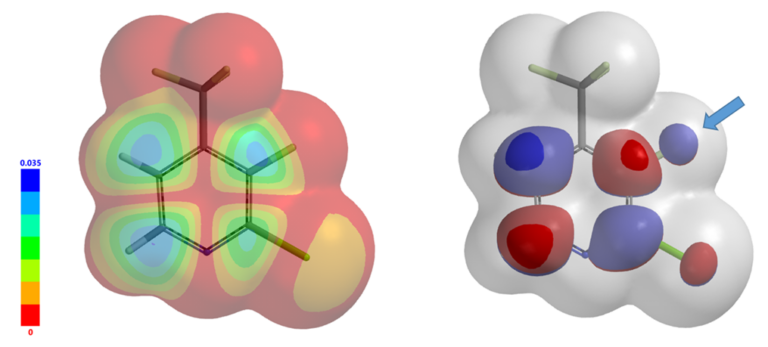
Figure 7. LUMO+1 map and LUMO+1/Electron Density map of compound I
References:
[1] Quantum Mechanics for Organic Chemists: An Experimentalist Approach. https://wuxibiology.com/resource/quantum-mechanics-for-organic-chemists/
[2] Spartan Tutorial and User’s Guide. Accessible within Spartan software and from https://www.wavefun.com/spartan-documentation
[3] W.W.K.R. Mederski, M. Lefort, M. Germann, D. Kux, Tetrahedron, 1999, 55, 12757.
[4] Reference 1, chapter 3: Application of Electrostatic Potential Map in Acidity Estimation.
[5] WO2019/147782, 2019, A1 p191, 194; US2012/77814, 2012, A1 p25; WO2011/25940, 2011, A1 p. 92.
[6] Reference 1, chapter 1: Application of LUMO Analysis in Nucleophilic Reactions.
[7] Reference 1, chapter 5: Sequential Cross Coupling Reactions of Polyhalogenated Heterocycles.
[8] QM Chapter 39: Unraveling Divergence in Haloselectivity of SNAr and Cross Coupling vs Halogen-Metal Exchange Reactions. https://wuxibiology.com/unraveling-divergence-in-haloselectivity-of-snar-and-cross-coupling-vs-halogen-metal-exchange-reactions/
[9] L. Tao, Y. Yue, J. Zhang, S.Y. Chen, X.Q. Yu, Helvetica Chimica Acta, 2008, 91, 1008.
[10] Reference 1, chapter 34: QM Analyses of Regioselectivity in Chan-Lam Reaction.
[11] L.C. Campeau, Q. Chen, D. Gauvreau, M. Girardin, K. Belyk, P. Maligres, G. Zhou, C.Z. Gu, W. Zhang, L.S. Tan, P. D. O’Shea, Org. Process Res. Dev. 2016, 20, 1476.