 Choose language
Choose language
< Return to main menu
 Choose language
Choose language

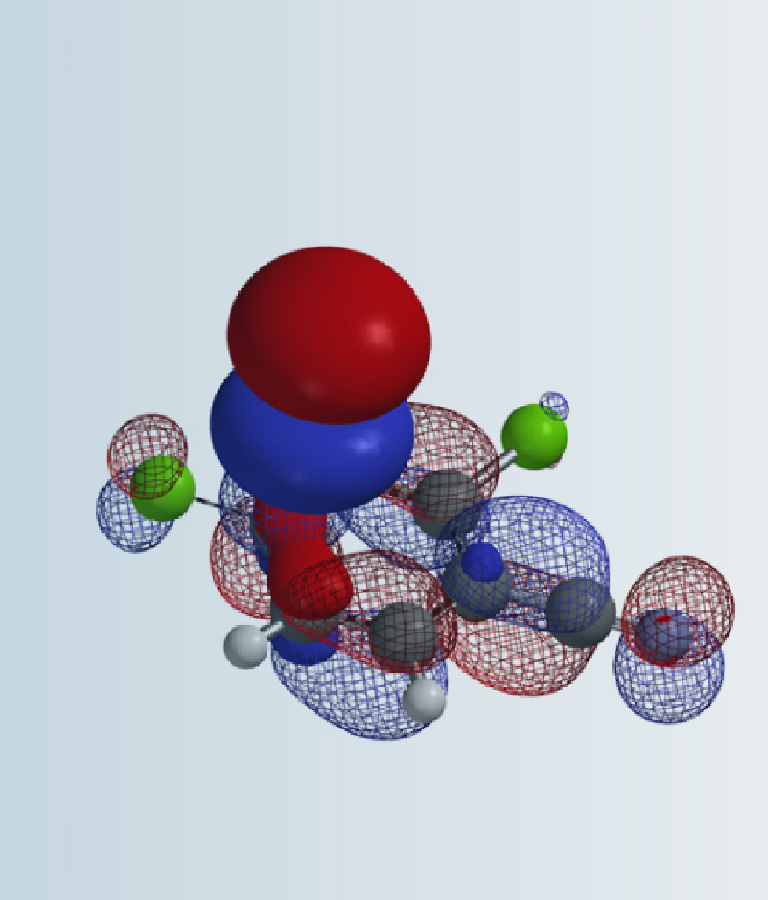
Previously we have introduced several cases to show how we apply HOMO and LUMO for predicting regio-selectivity on heterocyclic aromatic substrates. Today we will be looking at cases in which saturated ring systems are involved.
For the piperidine substrate (1) in Figure 1, deprotonation of the α-carbon to the ester followed by alkylation results in a quaternary chiral center in the product. Would the reaction be stereo-selective?
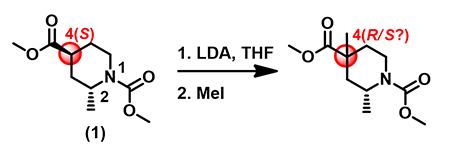
Figure 1. Alkylation of (2R,4S)-dimethyl 2-methylpiperidine-1,4-dicarboxylate.
A conformational analysis is often needed in such cases. Substituents on the saturated ring system could potentially exert substantial steric effects, biasing for one stereoisomer or the other.
In this article, we will present how we combine conformational analyses along with HOMO Map to provide a solid and straightforward way for stereo-selectivity prediction.
The “Conformer Distribution” task in Spartan is programmed to generate all reasonable conformations for the target and identify the most dominant one with the lowest energy. Figure 2-a shows the preferred conformation of the piperidine substrate (1). Both 1-N-methyl carbamate and 4-methyl ester groups are at equatorial positions as we generally recognize, whereas the 2-methyl group adopts an axial position.
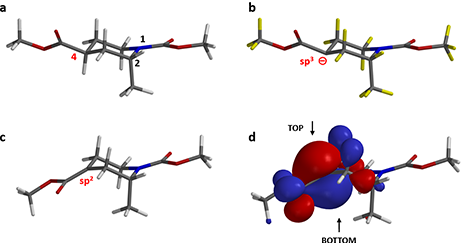
Figure 2. a. Dominant Conformation of substrate (1). b. Substrate (1) is deprotonated
at the α-carbon of methyl ester yielding a carbanion
intermediate. c. QM-minimized carbanionintermediate adopts the enolate form. d. HOMO lobes of the enolate intermediate.
The reaction proceeds in two steps. LDA deprotonates the α-carbon of methyl ester group, generating a carbanion intermediate, followed by alkylation with methyl iodide. Here we remove the hydrogen atom from the α-carbon at 4-position (Figure 2-b) to generate the chemical structure for QM calculations.
The QM-minimized carbanion intermediate structure is shown in Figure 2-c. The power of QM calculation is that it is capable of recognizing the electronic environment surrounding the carbanion and successfully correcting the hybridization status of the atom. The deprotonated α-carbon is sp2 hybridized, and the resultant enolate adopts a co-planar conformation.
Figure 2-d shows the HOMO on QM-minimized enolate intermediate. Clearly the largest HOMO lobes are on top and bottom of the plane of the enolate anion. It is anticipated that the 2-methyl group could limit access of the HOMO lobe from the bottom side through steric hindrance (1,3-diaxial interactions, a specific class of syn-pentane interactions). The question is: will it be completely or partially blocked?
Here, we would like to introduce the use of HOMO Map, along with “Inaccessible Markers”, a surface property presented in Spartan, to assist the analysis.
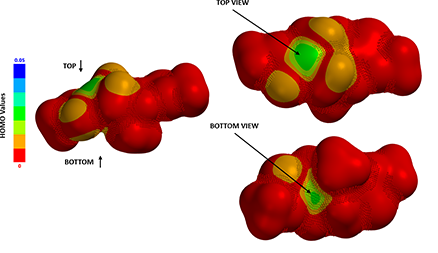
Figure 3. HOMO Map of the enolate intermediate.
By mapping HOMO energies onto electron density surface, we could generate the HOMO Map of the enolate intermediate, shown in Figure 3. Color near red indicates a low HOMO value with a low probability being attacked by electrophiles, whereas color near green-to-blue indicates higher HOMO energy values, suggesting the HOMO lobe is more accessible to the electrophilic reagent. Spartan also provides a useful tool, “Inaccessible Markers”, represented as dots distributed on surface, to distinguish regions that are not accessible.
Although HOMO values on top and bottom of the enolate anion are identical, as they are both in green colors, the bottom side is completely covered with inaccessible markers, which explicitly confirms what we anticipated: 2-methyl group brings in substantial 1,3-diaxial interactions, completely blocking the bottom side from being attacked. The methyl iodide can only approach from the top side, leading to 4-R stereoisomer as the sole product observed (Figure 4).
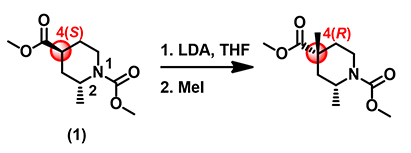
Figure 4. Alkylation of substrate (1) gives a 4-R stereo-selective product.
To summarize, for reactions involving saturated ring systems, we could use modeling tools to identify the dominant low-energy conformation. Subsequent QM calculations could further provide more accurate structural information along with frontier orbital distributions to locate where the reaction might occur. By taking the advantage of molecular surface properties, we can then visually and objectively evaluate the steric effects and predict the stereo-selectivity with high confidence.
Substrate (2) is a bicyclic trans-decalin with multiple substituents. Will deprotonation of a-carbon to the nitrile group followed by methylation provide the required quaternary chiral center (Figure 5-a)?
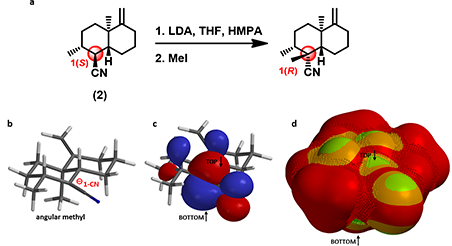
Figure 5. a. Alkylation reaction of substrate (2). b, c, and d. QM-minimized carbanion intermediate,
the corresponding HOMO lobes, and the HOMO Map of substrate (2), respectively.
QM-minimized carbanion intermediate structure (Figure 5-b) suggests a close-to-planar conformation at reaction center, in conjugation with the nitrile group. HOMO lobes on top and bottom of carbanion are in similar sizes (Figure 5-c). However, HOMO Map (Figure 5-d) reveals that the bottom side is completely covered with inaccessible markers, with access totally blocked by the angular methyl group. Experimentally only the 1-R methylated product is identified.
In the next chapter, we will apply all these FMO concepts to prospectively analyze a sequence of cross coupling reactions. Please stay tuned.
This article is written and edited by Guqin Shi, Wang Qiuyue, Pan Dong, Tommy Lai, and John S. Wai.
References:
[1] Warren J. Hehre (2003). A Guide to Molecular Mechanics and Quantum Chemical Calculations. Irvine, CA, USA: Wavefunction, Inc.
[2] Spartan '18 Tutorial and User's Guide (2019). Irvine, CA, USA: Wavefunction, Inc.
[3] R. W. Hoffmann, Angew. Chem. Int. Ed. Engl. 1992, 31, 1124.