 Choose language
Choose language
< Return to main menu
 Choose language
Choose language


Protection of nucleophilic amino group is an important strategy in organic synthesis. Among the protecting groups, Boc offers excellent stability towards a variety of nucleophilic and electrophilic reaction conditions while readily converted back to the parent amines under mild acidic conditions. Boc reaction of aliphatic amines with Boc anhydride is generally quite fast, however protection of weakly nucleophilic aromatic amines will take longer, may require heating or even addition of strong bases.
In 2006, Tirayut Vilaivan reported that alcoholic solvents greatly enhance the rate of Boc protection of aromatic amines at room temperature, and do not need to include any base in the reaction mixture (Figure 1). [2] Kinetics study with NMR spectroscopy showed that the reaction between p-toluidine and Boc anhydride in CD3OD is 70 fold faster than in CDCl3. We are curious in how alcohol catalyzes this reaction and decide to analyze this reaction further with QM calculations.
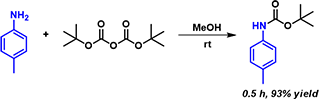
Figure 1. Methanol enhances the rate of Boc protection of aromatic amines
QM analyses quickly revealed potentially the presence of hydrogen bond between N-H of aniline and the carbonyl oxygen O1' in Boc anhydride (Figure 2A) that could lead to a 6-membered transition state. As such, a reaction energy profile calculation with the N-C2 distance to change from 3.2 Å to 1.4 Å (Figure 2A, blue dotted line), with a step size of 0.1 Å, and enabling hydrogen bonding interactions between N-H of aniline and O1' of Boc anhydride (Figure 2B, magenta dotted line) was set up and provided a calculated activation energy (ΔE) of 16.22 kcal/mol (Figure 4, red line).
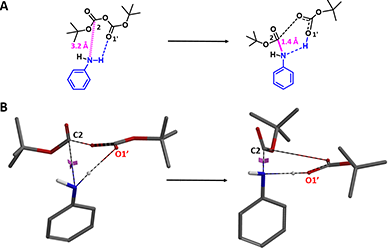
Figure 2. Aniline Boc protection modeling
For modeling of the reaction in the presence of methanol, we positioned it for potential non-covalent interactions with both the carbonyl oxygen O2' and the second N-H of aniline (Figure 3A, red dotted line). QM provided a calculated activation energy (ΔE) of 13.94 kcal/mol, 2.28 kcal/mol lower than in the absence of methanol (Figure 4, blue line), accounting for the experimental observation that the rate of aniline Boc protection proceeds faster in methanol.
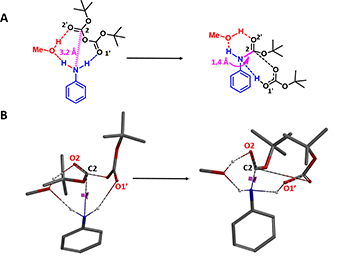
Figure 3. Including methanol in modeling of aniline Boc protection
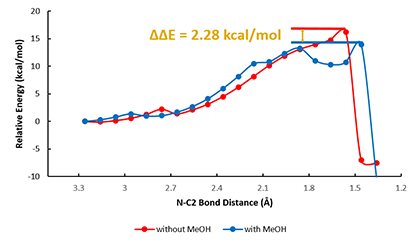
Figure 4. Reaction energy profiles of Boc protection of aniline in the absence (red) and in the presence (blue) of methanol
Let’s examine further the transition state structures TS1 and TS2 resulted from the calculations. In the transition state TS1 (Figure 5, without methanol), the distance between N-C2 is 1.56 Å, which is close to the bonding distance, meanwhile, the distance between N-H of aniline and the departing carbonyl oxygen O1' in Boc anhydride is 1.96 Å. This is within the range of hydrogen bonding and will have a stabilizing effect on transition state TS1.
In the transition state TS2 (Figure 5, with methanol), the distance between N-C2 is 1.46 Å and already bonded, and there is the same hydrogen bond between the N-H of aniline and the departing carbonyl oxygen O1' in Boc anhydride (1.88 Å). Furthermore, the included methanol, acts like a bridge, establishes hydrogen bonding to both the carbonyl oxygen O2' in Boc anhydride and the second N-H of aniline, with similar calculated bond distances of 1.83 Å. These supports our hypothesis that methanol introduces two additional hydrogen bonds to the transition state, further stabilizes it, lowers the activation energy, and speeds up the reaction.
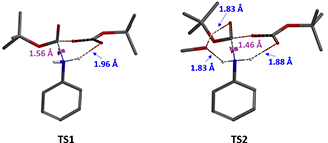
Figure 5. Transition states without methanol (TS1) and with methanol (TS2) in Boc protection of aniline
In the same paper, Vilaivan reported that in contrast to primary amines, with reactions complete from < 1 minutes to 2 hr, secondary aromatic amines react much slower, and will take 24 to 48 hr to go to completion in the same alcoholic solvent (Figure 6A). This observation could be accounted for with our proposed transition state (Figure 6B, TS3) losing the stabilization from the hydrogen bond between NH and alcohol.
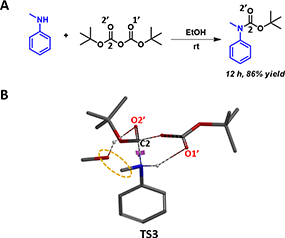
Figure 6. Boc protection of secondary aromatic amine
In summary, our QM calculations arrived with a useful model in Boc protection of primary amines, indicative that alcohol stabilizes the transition state with two additional hydrogen bonds, bridging the two reactants with a six-membered system, increasing the rate of reaction. The model could also account for the lack of acceleration with secondary amines. [3]
In organic synthesis, we learned to lactamize γ-amino esters in alcohol with a catalytic amount of acetic acid (Figure 7). Why do we add acetic acid? Will it protonate the amino group, making it less nucleophilic?
This article is written and edited by Renwei Zhang, Guqin Shi, Dong Pan, and John S. Wai.
References:
[1] Warren J. Hehre. A Guide to Molecular Mechanics and Quantum Chemical Calculations. Irvine, CA, USA: Wavefunction, Inc., 2003.
[2] T. Vilaivan Tetrahedron Lett. 2006, 47, 6739.
[3] Our model is also consistent with the use of water or aqueous organic solvent in primary amine Boc protection, see Chankeshwara, S.V. & Chakraborti, A.K. Org. Lett. 2006, 8, 3259.