 Choose language
Choose language
< Return to main menu
 Choose language
Choose language

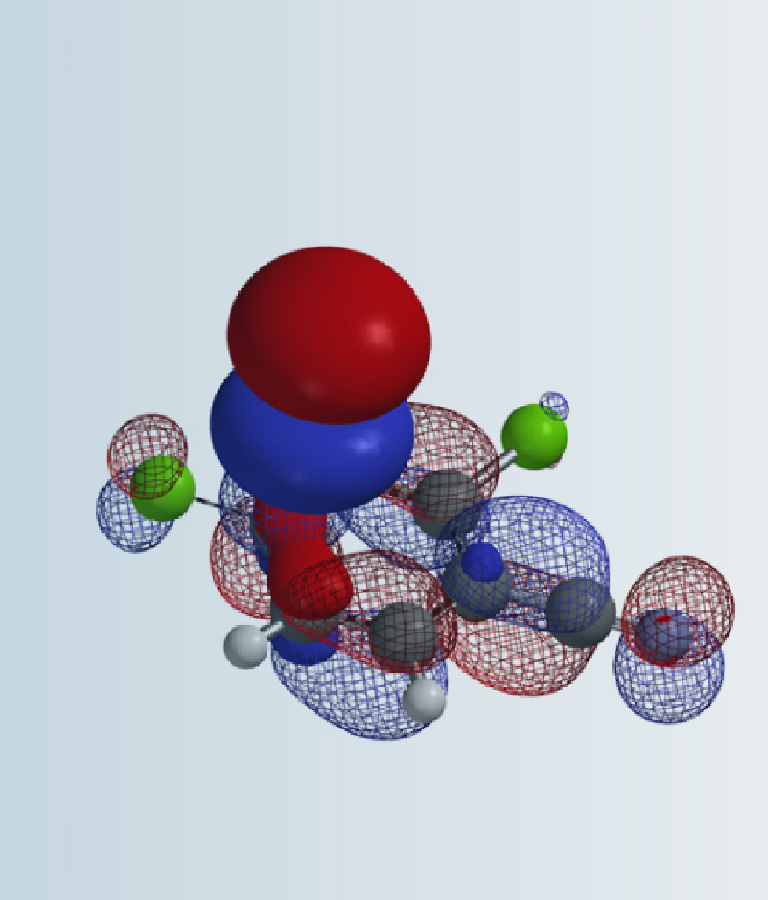
In synthetic planning of complex organic molecules, π-facial selectivity is often an important element to consider. The origin of such stereoselectivity has been attributed to a number of factors such as electrostatics, steric, hyperconjugation effects, and a mysterious Factor X [1,2]. In this chapter we will discuss what is Factor X, how we could use QM to model, analyze, quantify, and visualize this important controlling element in stereoselectivity.
It is well-known that peracid epoxidation of norbornene results in highly selective exo product, as shown in Figure 1.
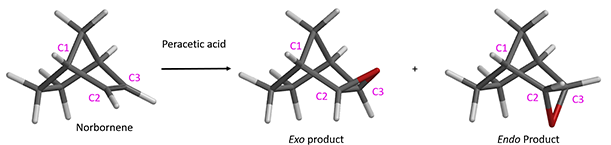
Figure 1. Epoxidation of norbornene
We modeled this Prilezhaev reaction with peracetic acid attacking from both faces of norbornene. Shown on Figure 2 is the approach from bottom face, leading to the formation of endo product. Geometry and energy were calculated as O-C2 distance varies from 3.2 Å to 1.9 Å, with a step size of 0.1 Å, assuming synchronized O-C2 and O-C3 bond formations.
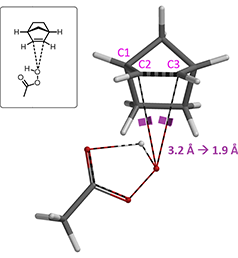
Figure 2. Model of epoxidation of norbornene with peracetic acid attacking from bottom face
Reaction energy profile calculations (Figure 3) suggested activation energies ΔE of 26.95 and 22.33 kcal/mol for peracetic acid attacking from bottom and top faces, respectively, to provide the corresponding endo and exo products. A difference (ΔΔE) of 4.62 kcal/mol indicates a highly selective reaction, producing mainly the exo product.
What causes the energy difference of 4.62 kcal/mol?
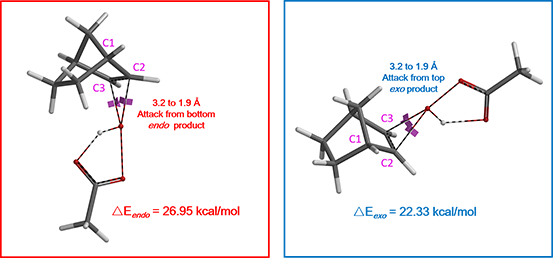
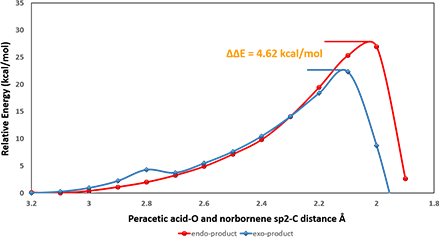
Figure 3. Reaction energy profiles of peracetic acid attacking from bottom/top faces giving endo/exo products, respectively
In stereochemistry, every three non-collinear atoms of a molecule determines a plane. When two such planes intersect, the angle between them is defined as a dihedral angle, or a torsion angle.
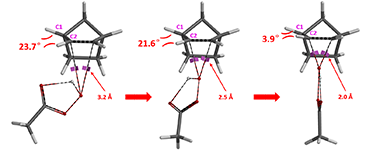
Careful inspection on the geometries at initial states (with O-C bond length at 3.2 Å), mid-way (2.5 Å), and the transition states (2.0 Å or 2.1 Å), showed that as epoxidation proceeds, C1-H and C2-H bonds approach each other and build up steric congestion when peracid attacks from bottom face (Figure 4). In contrast, C1-H and C2-H move away from each other and relieve steric stress when being attacked from top face.
Attack from bottom face providing endo product:
Attack from top face providing exo product:
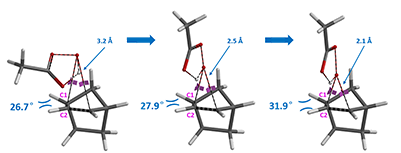
Figure 4. Dihedral angle changes during Prilezhaev reaction progress
In summary, it is the difference in torsional strain that controls the selectivity observed in peracetic epoxidation of norbornene. For what was once being considered to be a mystery (the Factor X), QM analysis shows us that it is comprehensible, predictable, and quantifiable.
For the following nucleophilic opening of epoxide 1, if we apply the above torsional strain analysis, shall we expect 2 or 3 to be the major product?
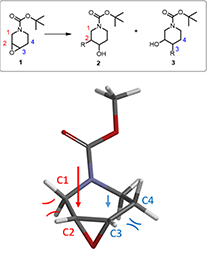
Figure 5. Ring-opening reaction of epoxide 1
This article is written and edited by Qiuyue Wang, Guqin Shi, and John S. Wai.
References:
[1] a) R. Huisgen, P. H. J. Ooms, M. Mingin, N.L. Allinger., J. Am. Chem. Soc. 1980, 102, 3951. b) R. Huisgen., Pure Appl. Chem., 1981, 53, 171.
[2] H. Wang & K. N. Houk., Chem Sci. 2014, 5, 462.
[3] R. D. Bach, C. Canepa, J. E. Winter, P. E. Blanchette., J. Org. Chem. 1997, 62, 5191.